Current and Past Research Themes
Engineering the Surfaces of Native Biomolecular Condensate Assemblies
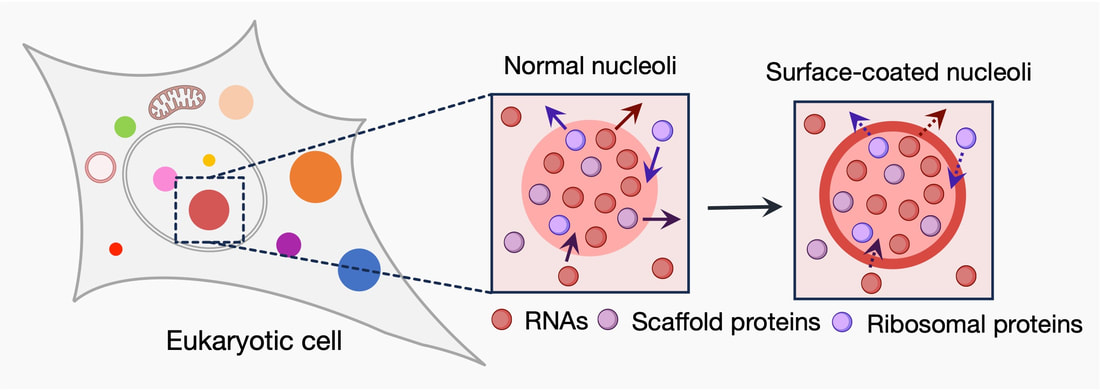
In my current research, I create DNAs that when expressed by living cells translate to light-responsive proteins. The intracellular compartmentalization of such light-responsive proteins can be modulated “on demand” from the inside of membraneless organelles to their surface. This space-switching system alters the surface structure and biochemical output of native membraneless organelles such as nucleoli and stress granules. In principle, controlling the output of RNA synthesized in the nucleoli may resolve the abnormal RNA production commonly found in the nucleoli of cancer and neurodegenerative diseases.
In my current research, I create DNAs that when expressed by living cells translate to light-responsive proteins. The intracellular compartmentalization of such light-responsive proteins can be modulated “on demand” from the inside of membraneless organelles to their surface. This space-switching system alters the surface structure and biochemical output of native membraneless organelles such as nucleoli and stress granules. In principle, controlling the output of RNA synthesized in the nucleoli may resolve the abnormal RNA production commonly found in the nucleoli of cancer and neurodegenerative diseases.
Liquid-Liquid Phase Separation of Nonionic Polymers
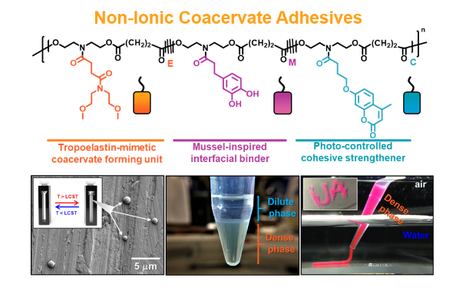
Coacervation is macroscopic phase separation of a solution to form two distinct fluid-fluid phases, dilute and dense. The dense phase of the coacervate has unique characteristics such as high density, low viscosity, and low interfacial energy. Coacervation allows polymers with lower interfacial energy to stick to wet surfaces effortlessly. All synthetic coacervates reported to date are prepared from complexing oppositely charged polymers. Due to the ionic nature of these polymers, the coacervation was only achieved near-neutral pH and with a highly ionic solution. These restricted conditions limited the application of synthetic coacervates in dynamic environments such as biological systems. The concept of ‘charge-free’ coacervate adhesives that can self-coacervate in all ranges of pH and ionic strength are still missing in the scientific community. This unmet need was the motivation for me to develop non-ionic coacervates. Preparation of non-ionic and single component coacervates has significant advantages over the charged coacervates since their formation does not require optimization of the molar ratio of two or more components. The thesis describes the creation of a synthetic mimic of tropoelastin, a commonly found protein in mammals that undergoes non-ionic self-coacervation. Sandcastle worm silk and mammalian proteins-inspired coacervates demonstrate strong underwater adhesion. Coacervation also allows to encapsulate growth factors and blood clotting factors and facilitate wound healing. The combination of coacervation and hydrophobic polymers has the potential to work efficiently as an ‘ideal tissue sealant’ for internal organs. Coacervation will provide strong interfacial interactions and the hydrophobic polymer will provide cohesive strength and structural integrity to the tissue adhesive. The initial data from the ex vivo hepatic hemorrhage model experiments using this approach is promising.
Coacervation is macroscopic phase separation of a solution to form two distinct fluid-fluid phases, dilute and dense. The dense phase of the coacervate has unique characteristics such as high density, low viscosity, and low interfacial energy. Coacervation allows polymers with lower interfacial energy to stick to wet surfaces effortlessly. All synthetic coacervates reported to date are prepared from complexing oppositely charged polymers. Due to the ionic nature of these polymers, the coacervation was only achieved near-neutral pH and with a highly ionic solution. These restricted conditions limited the application of synthetic coacervates in dynamic environments such as biological systems. The concept of ‘charge-free’ coacervate adhesives that can self-coacervate in all ranges of pH and ionic strength are still missing in the scientific community. This unmet need was the motivation for me to develop non-ionic coacervates. Preparation of non-ionic and single component coacervates has significant advantages over the charged coacervates since their formation does not require optimization of the molar ratio of two or more components. The thesis describes the creation of a synthetic mimic of tropoelastin, a commonly found protein in mammals that undergoes non-ionic self-coacervation. Sandcastle worm silk and mammalian proteins-inspired coacervates demonstrate strong underwater adhesion. Coacervation also allows to encapsulate growth factors and blood clotting factors and facilitate wound healing. The combination of coacervation and hydrophobic polymers has the potential to work efficiently as an ‘ideal tissue sealant’ for internal organs. Coacervation will provide strong interfacial interactions and the hydrophobic polymer will provide cohesive strength and structural integrity to the tissue adhesive. The initial data from the ex vivo hepatic hemorrhage model experiments using this approach is promising.
Molecular Structure and Underwater Adhesion of Bioinspired Polymers
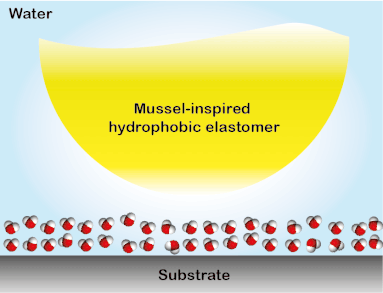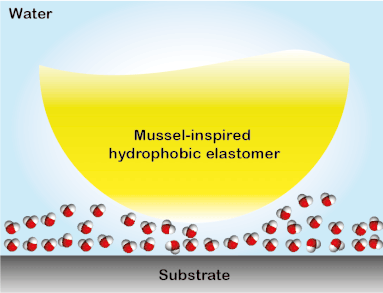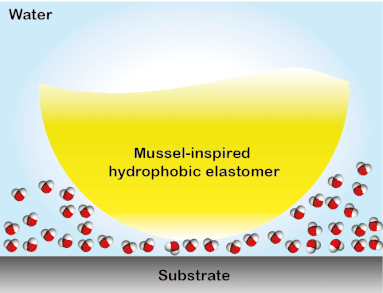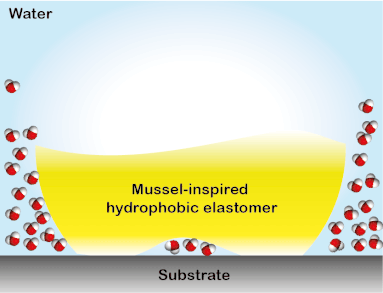
Wound closure techniques such as sutures and staples have drawbacks such as they pierce surrounding tissues, require debris removal, and they often lead to formation scars. To overcome this collateral damage, a library of elastomeric adhesives that conjoin wounded tissues even in the presence of blood was created. The elastomers were designed to absorb biologically and are non-toxic, therefore, they have the potential to replace conventional wound closure procedures (Biomacromolecules 2019, 20, 2577). This tissue sealant material was developed in a stepwise process. First, using the multifunctional polyester array, an investigation on the key parameters that govern underwater adhesion was performed. It was discovered that for a solvent-free adhesive system suitable for hemorrhage control, low glass transition temperature, hydrophobicity, and apt viscosity are required to obtain high work of adhesion underwater (Adv. Mater. Interfaces 2017, 4, 1700506). A similar polymer system was used to study the interfacial binding behavior of bioinspired functional groups. The study led to the development of the strongest solvent-free underwater adhesive reported till date and a ground-breaking understanding of the mechanism of bio-inspired underwater adhesion. It was observed that the DOPA is not effective in removing the interstitial water, contrary to the commonly held opinion in this field. Instead, the hydrophobic groups present in the elastomers partially remove the interstitial water to facilitate the interfacial contacts underwater. The discovery of the synergy between catechol binding and hydrophobicity in enabling the mussel-inspired soft adhesive elastomer to stick underwater provided a framework for designing materials for applications in tissue adhesion and moist-skin wearable electronics (ACS Cent. Sci. 2018, 4, 1420-1429). The generation 1 adhesives were featured in a blog by ‘insidescience.org’ and a popular science show ‘Nature Knows Best’ in Amazon Prime and Hulu.
Wound closure techniques such as sutures and staples have drawbacks such as they pierce surrounding tissues, require debris removal, and they often lead to formation scars. To overcome this collateral damage, a library of elastomeric adhesives that conjoin wounded tissues even in the presence of blood was created. The elastomers were designed to absorb biologically and are non-toxic, therefore, they have the potential to replace conventional wound closure procedures (Biomacromolecules 2019, 20, 2577). This tissue sealant material was developed in a stepwise process. First, using the multifunctional polyester array, an investigation on the key parameters that govern underwater adhesion was performed. It was discovered that for a solvent-free adhesive system suitable for hemorrhage control, low glass transition temperature, hydrophobicity, and apt viscosity are required to obtain high work of adhesion underwater (Adv. Mater. Interfaces 2017, 4, 1700506). A similar polymer system was used to study the interfacial binding behavior of bioinspired functional groups. The study led to the development of the strongest solvent-free underwater adhesive reported till date and a ground-breaking understanding of the mechanism of bio-inspired underwater adhesion. It was observed that the DOPA is not effective in removing the interstitial water, contrary to the commonly held opinion in this field. Instead, the hydrophobic groups present in the elastomers partially remove the interstitial water to facilitate the interfacial contacts underwater. The discovery of the synergy between catechol binding and hydrophobicity in enabling the mussel-inspired soft adhesive elastomer to stick underwater provided a framework for designing materials for applications in tissue adhesion and moist-skin wearable electronics (ACS Cent. Sci. 2018, 4, 1420-1429). The generation 1 adhesives were featured in a blog by ‘insidescience.org’ and a popular science show ‘Nature Knows Best’ in Amazon Prime and Hulu.
Volume Phase Transition of Polymers at Low Concentration
Probing volume phase transition behavior of superdiluted polymer solutions both micro- and macroscopically still persists as an outstanding challenge. In this regard, we have explored 4 × 4 spectral Mueller matrix measurement and its inverse analysis for excavating the microarchitectural facts about stimuli responsiveness of “smart” polymers. Phase separation behavior of thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) and pH responsive poly(N,N-(dimethylamino)ethyl methacrylate) (PDMAEMA) and their copolymers were analyzed in terms of Mueller matrix derived polarization parameters, namely, depolarization (Δ), diattenuation (d), and linear retardance (δ). The Δ, d, and δ parameters provided useful information on both macro- and microstructural alterations during the phase separation. Additionally, the two step action ((i) breakage of polymer–water hydrogen bonding and (ii) polymer–polymer aggregation) at the molecular microenvironment during the cloud point generation was successfully probed via these parameters. It is demonstrated that, in comparison to the present techniques available for assessing the hydrophobic–hydrophilic switch over of simple stimuli-responsive polymers, Mueller matrix polarimetry offers an important advantage requiring a few hundred times dilute polymer solution (0.01 mg/mL, 1.1–1.4 μM) at a low-volume format.
Molecular Interactions of Counterions with Polyelectrolytes
Effects of counterions of side chain amino acid based polyelectrolytes (PEs) on the solubility in aqueous medium, pH responsiveness, thermal properties, and ionic conductivities have been appraised. Deprotection of the tert-butyl carbamate (Boc) group from poly(Boc-l-leucine methacryloyloxyethyl ester) [P(Boc-l-Leu-HEMA)] was carried out to produce PE with trifluoroacetate as an associative counteranion (1a). PEs with bis(trifluoromethylsulfonyl)imide and hexafluorophosphate counteranion were prepared through anion exchange reactions of 1a. Protonation of the neutralized polymer (2) obtained from 1a, followed by anion exchange, leads to the production of miscellaneous PEs bearing different counteranions, such as tetrafluoroborate, trifluoromethanesulfonate, chloride, and nitrate. Differential scanning calorimetry traces of the PEs reveal that the comparatively larger and weakly coordinated counteranions require less thermal energy to dissociate, and thus, the glass transition temperature (Tg) of the PEs fall off with an increase in the size of the counteranion. A remarkable conductivity of 2.1 mS/cm was obtained in deionized water when Cl– acted as the counteranion. Steric and electronic factors of the counteranion induce a change of transition pH in different PEs, although the chiroptical nature was retained, as confirmed by circular dichroism spectroscopy.
Probing volume phase transition behavior of superdiluted polymer solutions both micro- and macroscopically still persists as an outstanding challenge. In this regard, we have explored 4 × 4 spectral Mueller matrix measurement and its inverse analysis for excavating the microarchitectural facts about stimuli responsiveness of “smart” polymers. Phase separation behavior of thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) and pH responsive poly(N,N-(dimethylamino)ethyl methacrylate) (PDMAEMA) and their copolymers were analyzed in terms of Mueller matrix derived polarization parameters, namely, depolarization (Δ), diattenuation (d), and linear retardance (δ). The Δ, d, and δ parameters provided useful information on both macro- and microstructural alterations during the phase separation. Additionally, the two step action ((i) breakage of polymer–water hydrogen bonding and (ii) polymer–polymer aggregation) at the molecular microenvironment during the cloud point generation was successfully probed via these parameters. It is demonstrated that, in comparison to the present techniques available for assessing the hydrophobic–hydrophilic switch over of simple stimuli-responsive polymers, Mueller matrix polarimetry offers an important advantage requiring a few hundred times dilute polymer solution (0.01 mg/mL, 1.1–1.4 μM) at a low-volume format.
Molecular Interactions of Counterions with Polyelectrolytes
Effects of counterions of side chain amino acid based polyelectrolytes (PEs) on the solubility in aqueous medium, pH responsiveness, thermal properties, and ionic conductivities have been appraised. Deprotection of the tert-butyl carbamate (Boc) group from poly(Boc-l-leucine methacryloyloxyethyl ester) [P(Boc-l-Leu-HEMA)] was carried out to produce PE with trifluoroacetate as an associative counteranion (1a). PEs with bis(trifluoromethylsulfonyl)imide and hexafluorophosphate counteranion were prepared through anion exchange reactions of 1a. Protonation of the neutralized polymer (2) obtained from 1a, followed by anion exchange, leads to the production of miscellaneous PEs bearing different counteranions, such as tetrafluoroborate, trifluoromethanesulfonate, chloride, and nitrate. Differential scanning calorimetry traces of the PEs reveal that the comparatively larger and weakly coordinated counteranions require less thermal energy to dissociate, and thus, the glass transition temperature (Tg) of the PEs fall off with an increase in the size of the counteranion. A remarkable conductivity of 2.1 mS/cm was obtained in deionized water when Cl– acted as the counteranion. Steric and electronic factors of the counteranion induce a change of transition pH in different PEs, although the chiroptical nature was retained, as confirmed by circular dichroism spectroscopy.